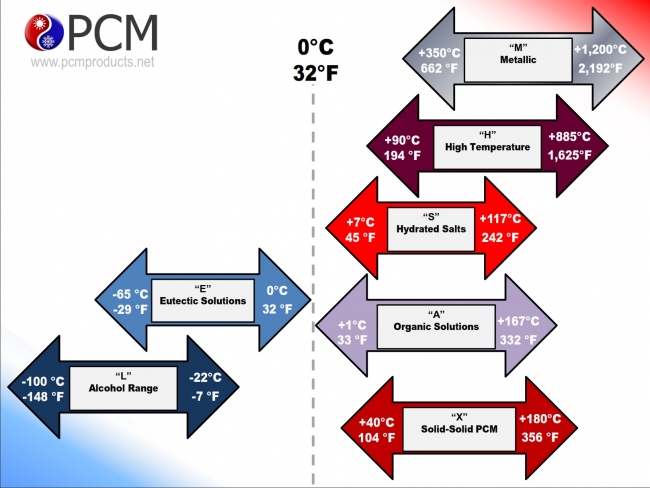
favorable materials for thermal storage as phase change with their properties
Materials used for thermal storage as phase change materials (PCMs) play a crucial role in applications such as energy storage, temperature regulation, and thermal management. Here are some commonly used PCMs along with their properties:
-
Paraffin Wax:
- Melting Point: Typically in the range of 47 to 64 degrees Celsius.
- Heat of Fusion: Around 180 to 220 J/g.
- Thermal Conductivity: Relatively low, providing good insulation.
-
Salt Hydrates (e.g., Sodium Sulfate Decahydrate):
- Melting Point: Varies depending on the specific salt hydrate, commonly in the range of 30 to 100 degrees Celsius.
- Heat of Fusion: Can be high, providing substantial energy storage.
- Thermal Conductivity: Moderate.
-
Glycerol:
- Melting Point: About 18 degrees Celsius.
- Heat of Fusion: Approximately 252 J/g.
- Thermal Conductivity: Moderate.
-
Calcium Chloride Hexahydrate:
- Melting Point: Around 29 degrees Celsius.
- Heat of Fusion: Approximately 83 J/g.
- Thermal Conductivity: Moderate.
-
Eutectic Mixtures (e.g., Binary Mixtures of Fatty Acids):
- Melting Point: Can be tailored by combining different materials.
- Heat of Fusion: Depends on the specific mixture.
- Thermal Conductivity: Varies based on the components.
-
Water (for High-Temperature Applications):
- Melting and Boiling Points: 0 degrees Celsius and 100 degrees Celsius, respectively.
- Heat of Fusion: Around 334 J/g.
- Thermal Conductivity: Relatively high.
-
Metal Alloys (e.g., Indium-Gallium Eutectic):
- Melting Point: Varies depending on the alloy.
- Heat of Fusion: Depends on the specific alloy.
- Thermal Conductivity: Generally high.
created by ChatGPT AI tool